Answer:

Explanation: The question asks to write the balanced equation for the decomposition of methanol to form carbon monoxide and hydrogen gas.
In a chemical equation, we write the reactants on the left side and the products on right side and put an arrow between them.
Methanol has one carbon, one oxygen and four hydrogens. Carbon monoxide has one carbon and one oxygen and hydrogen gas has two hydrogens. The unbalanced equation will be:
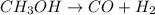
Carbon and oxygen are balanced but hydrogen is not. Left side has four hydrogen whereas right side has only two. So, to make it balanced, we need to multiply
 by 2. The balanced equation will be:
by 2. The balanced equation will be:
