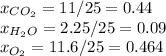Answer:
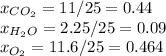
Step-by-step explanation:
First we need to to set and balance the equation:
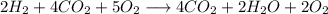
With this we can balance the equation with our molar fractions, and we will asume 1 mole of mixture at the beginning for practical purposes:
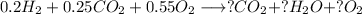
With the equation we need to balance with the limit reactant, that is CO:
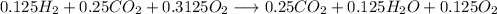
Now we just need to operate with the fractions that we have:
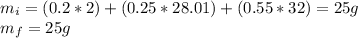
We proceed to calculate the mass of each product at the end with the moles of the balanced equation, remember that we had an excess of oxygen at the beginning, so we need to add that excess at the end:
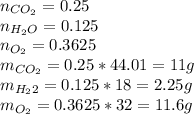
Now that we have the masses of each product, and the total mass, we can calculate the fraction: