Answer : The pH after 30 mL of NaOH added will be 11.96
Explanation : Given,
Concentration of acetic acid = 0.10 M
Volume of acetic acid = 25 mL = 0.025 L (conversion used : 1 L = 1000 mL)
Concentration of NaOH = 0.10 M
Volume of NaOH = 30 mL = 0.030 L
First we have to calculate the moles of
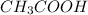 and
and
 .
.
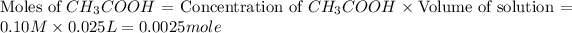
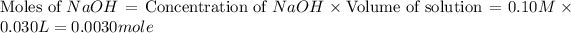
The balanced chemical reaction is,
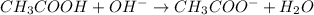
Initial moles 0.0025 0.0030 0
At eqm. moles 0 (0.0030-0.0025) 0.0025
= 0.0005
Now we have to calculate the hydroxide ion concentration.
![[OH^-]=\frac{\text{Moles of }OH^-}{\text{Total volume}}](https://img.qammunity.org/2020/formulas/chemistry/college/3zqiuc9l94daz9gzt5trf5vudarmzs4sil.png)
![[OH^-]=(0.0005mole)/((25+30)mL)=9.09* 10^(-6)mole/mL=9.09* 10^(-3)M](https://img.qammunity.org/2020/formulas/chemistry/college/dk9gafqmkoqjhvi5ztk9wumr3f3g13vupx.png)
Now we have to calculate the hydrogen ion concentration.
![[H^+][OH^-]=K_w](https://img.qammunity.org/2020/formulas/chemistry/college/zfeo9cpjxuenzlbyzisejh98ynub0dkhkr.png)
![[H^+]* 9.09* 10^(-3)=1.0* 10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/college/ofu9t40y64tt9h3xhvjyea1h200ndepiip.png)
![[H^+]=1.1* 10^(-12)M](https://img.qammunity.org/2020/formulas/chemistry/college/rqr99u0e71jxf98j5yqoe8s733w4gfelam.png)
Now we have to calculate the pH.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
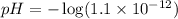
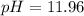
Therefore, the pH after 30 mL of NaOH added will be 11.96