Answer : The identity of the metal is, iron (Fe).
Explanation :
As we are given that:
65.57 % Cl by mass means that 65.57 gram of chlorine present in 100 grams of compound.
Mass of compound = 100 g
Mass of chlorine = 65.57 g
Mass of metal = 100 - 65.57 = 34.43 g
First we have to calculate the moles of chlorine.
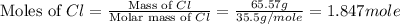
The given formula of compound is,
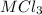
The ratio of M : Cl = 1 : 3
Now we have to calculate the moles of metal.
As, 3 moles of Cl combine with 1 mole of metal
So, 1.847 mole of Cl combine with
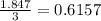 mole of metal
mole of metal
Now we have to calculate the molar mass of metal.
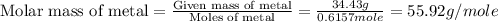
The molar mass of metal is 55.92 g/mole. From this we conclude that the metal is iron (Fe). So, the formula will be,
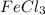
Hence, the identity of the metal is, iron (Fe).