Answer:
-2000 J (heat lost)
Step-by-step explanation:
We can solve the problem by using the 1st law of thermodynamics:
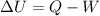
where
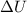 is the change in internal energy of a system
is the change in internal energy of a system
Q is the heat absorbed by the system
W is the work done by the system
In this situation, we have
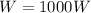 (work done by the student)
(work done by the student)
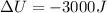 (decrease in internal energy)
(decrease in internal energy)
So the heat is
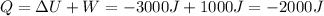
And the negative sign means the student has lost heat.