Answer: The kilograms of water must evaporate from 8kg of a 25% salt solution to produce 40% salt solution is 3 kg.
Step-by-step explanation:
According to the ratio and proportion:
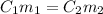
where,
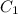 = concentration of ist solution = 25%
= concentration of ist solution = 25%
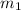 = mass of ist solution = 8 kg
= mass of ist solution = 8 kg
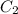 = concentration of second solution = 40%
= concentration of second solution = 40%
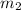 = mass of second solution = ? kg
= mass of second solution = ? kg
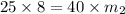
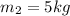
Thus the final solution must have a mass of 5 kg , i.e (8-5)= 3 kg of mass must be evaporated.
Therefore, the mass that must be evaporated from 8kg of a 25% salt solution to produce 40% salt solution is 3 kg.