Answer:
2.8212 g
Step-by-step explanation:
The reaction of copper sulfate with zinc is shown below as:
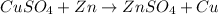
Given that :
Amount of zinc = 2.9 g
Molar mass of zinc = 65.38 g/mol
The formula for the calculation of moles is shown below:

Thus, molesare:
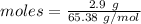
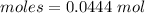
From the reaction,
1 mole of zinc on reaction forms 1 mole of copper
Thus,
0.0444 moles of zinc on reaction forms 0.0444 mole of copper
Mass of copper = moles×Molar mass
Molar mass of copper = 63.54 g/mol
Mass of copper = 0.0444 ×63.54 g = 2.8212 g