Answer:
2.49 V.
Step-by-step explanation:
The formula which relates the equilibrium condition in the electrochemical cell is,
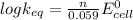
Given that the number of electron is 3 and the value of
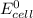 is 0.652 V.
is 0.652 V.
Now put these values in above equation.

Therefore, the value of Keq for an electrochemical reaction is 2.49 V.