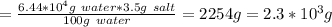Answer:
Amount of salt needed is around 2.3*10³ g
Step-by-step explanation:
The salt content in sea water = 3.5 %
This implies that there is 3.5 g salt in 100 g sea water
Density of seawater = 1.03 g/ml
Volume of seawater = volume of tank = 62.5 L = 62500 ml
Therefore, the amount of seawater required is:
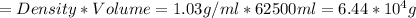
The amount of salt needed for the calculated amount of seawater is: