Answer:
66.57%
Step-by-step explanation:
The decomposition reaction of calcium carbonate is shown below as:

Calculation of moles of
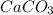 :
:
Amount = 3.32 g
Molar mass of
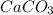 = 100 g/mol
= 100 g/mol
The formula for the calculation of moles is shown below:
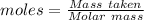
Thus, moles are:
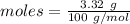
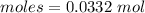
According to reaction,
0.0332 moles of
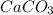 decomposes to yield 0.0332 moles of
decomposes to yield 0.0332 moles of
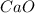
Theoretical yield = 0.0332 moles
Calculation of moles of
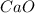 formed as:
formed as:
Amount = 1.24 g
Molar mass of
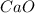 = 56 g/mol
= 56 g/mol
The formula for the calculation of moles is shown below:
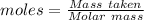
Thus, moles are:
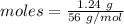
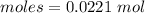
Experimental yield = 0.0221 moles
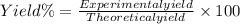
Thus,
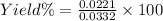
Percent yield = 66.57%