Answer:
The net entropy change of the entire system is increasing during a real isothermal expansion.
Step-by-step explanation:
The net entropy of a system is always zero for reversible process, and it is positive for irreversible process. It is also important to notice that the net entropy is the change of total entropy, that means.
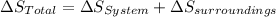
In a reversible isothermal process the total entropy change is 0, where the increase of entropy of the system has the same magnitude as the decrease of the entropy of the surroundings.
But in a real isothermal process the magnitude of the entropy lost by the surroundings is less than the magnitude of entropy gained by the system, thus we have irreversible process so the net entropy is always positive.
In case of a gas for example where we have isothermal expansion, the work done is by the gas, causing a heat loss by the surroundings. It is that temperature difference that will make the entropy lost by the surroundings less than the magnitude of entropy gained by the gas.