Answer: When

Explanation:
According to first law of thermodynamics, energy can neither be created nor be destroyed. It can only be transformed from one form to another.
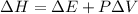
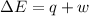
 = change in enthalpy
= change in enthalpy
P = pressure
 = change in volume
= change in volume
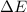 =Change in internal energy
=Change in internal energy
q = heat absorbed or released
w = work done on or by the system
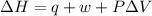
Thus for
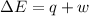 ,
,

The heat evolved or absorbed by the system in a physical or chemical process, equal the change in enthalpy of the system when
