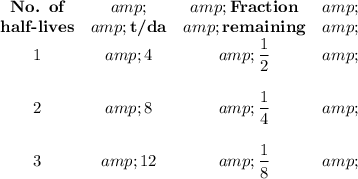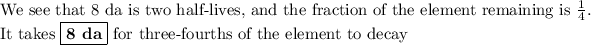Answer:
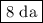
Step-by-step explanation:
The question will be easier to solve if we interpret it as, " How long will it take until one-fourth of a sample of the element remains,?"
The half-life of the element is the time it takes for half of it to decay.
After one half-life, half (50 %) of the original amount will remain.
After a second half-life, half of that amount (25 %) will remain, and so on.
We can construct a table as follows: