Step-by-step explanation:
Polar covalent bonding is the type of the chemical bond in which the pair of the electrons is unequally shared between the two atoms. As a result, the atom with higher value of electronegativity acquires a slightly negative charge and the atom with lower value of electronegativity acquires a slightly positive charge.
In the molecule of
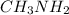 , the bond which is closest to ionic end of bond spectrum is N-H bond because the nitrogen atom is more electronegative than hydrogen and is ionic in nature.
, the bond which is closest to ionic end of bond spectrum is N-H bond because the nitrogen atom is more electronegative than hydrogen and is ionic in nature.
In the molecule of
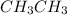 , the bond which is closest to ionic end of bond spectrum is no one because there is not much difference between carbon and hydrogen for the bond to be said as ionic.
, the bond which is closest to ionic end of bond spectrum is no one because there is not much difference between carbon and hydrogen for the bond to be said as ionic.
In the molecule of
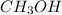 , the bond which is closest to ionic end of bond spectrum is O-H bond because the oxygen atom is more electronegative than hydrogen and is ionic in nature.
, the bond which is closest to ionic end of bond spectrum is O-H bond because the oxygen atom is more electronegative than hydrogen and is ionic in nature.