Answer:
12.24%
Step-by-step explanation:
It is given that the handbook gives the density of the liquid as
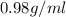
And on the based of lab measurement the density is 1.10 g/ml
Now we have to find the % error in the density measurement
So percentage error
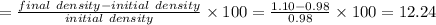 %
%
So the percentage error in the density measurement is 12.24%