Step-by-step explanation:
It is known that number of moles is the amount of mass present in a given molar mass of the substance.
Mathematically, No. of moles =

We are given that mass of
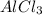 is 0.255 g and molar mass of
is 0.255 g and molar mass of
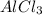 is 133.34 g/mol.
is 133.34 g/mol.
Therefore, number of moles of
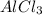 is calculated as follows.
is calculated as follows.
No. of moles =

=
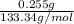
=
 mol
mol
Since, there are three chlorine atoms present in one mole of
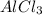 . Therefore, moles of chlorine ions present in given 0.255 g will be calculated as follows.
. Therefore, moles of chlorine ions present in given 0.255 g will be calculated as follows.
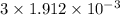 mol
mol
=
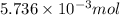
Thus, we can conclude that
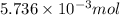 chloride ions are in 0.2550 g of aluminum chloride using
chloride ions are in 0.2550 g of aluminum chloride using
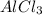 's molar mass of 133.34 g/mol.
's molar mass of 133.34 g/mol.