Answer:
Structures of products has been shown below
Step-by-step explanation:
- In claisen condensation, two ester molecules combines with each other through nucleophilic acyl substitution resulting formation of a
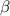 -ketoester.
-ketoester. - At first, one ester molecule gets deprotonated in presence of a strong base e.g. NaOEt yielding a carbanion.
- The carbanion then gives nucleophilic acyl substitution reaction with another ester molecule to replace alkoxy group present in ester group
- General reaction mechanism of claisen condensation along with products of given esters are shown below.