Answer:
324.18 g/mol
Step-by-step explanation:
Let the molecular mass of the antimalarial drug, Quinine is x g/mol
According to question,
Nitrogen present in the drug is 8.63% of x
So, mass of nitrogen =
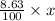
Also, according to the question,
2 atoms are present in 1 molecule of the drug.
Mass of nitrogen = 14.01 amu = 14.01 g/mol (grams for 1 mole)
So, mass of nitrogen = 14.01×2 = 28.02
These 2 must be equal so,
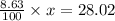
solving for x, we get:
x = 324.18 g/mol