Answer:
so total 6 spectral lines are possible here
Step-by-step explanation:
Since the electron is at excited state of n= 4
so here we have electron from nth excited to lower possible state
here the lower most energy state is ground state
so we will have
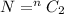
here we know
n = 4
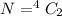
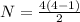
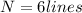
so total 6 spectral lines are possible here