Answer:
246.28 K
Step-by-step explanation:
The total energy of one mole of gas molecules can be calculated by the formula given below
E =
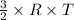
Where R is gas constant and T is absolute temperature.
Put the value of R as 8.314 and temperature as 245 , we get
E =
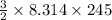
= 3055.4 J
Add 16 j to it
Total energy of gas molecules = 3055.4 + 16 = 3071.4 J.
If T be the temperature after addition of energy then
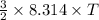 = 3071.4
= 3071.4
T =
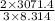
T = 246.28 K