Answer : The volume of water needed to recrystallize 0.900 g of benzoic acid is 13.24 mL.
Explanation :
For the complete recrystalization, the amount of hot water should be such that, the benzoic acid is completely soluble in it.
As we are given that the solubility of benzoic acid in hot water is 68.0 g/L. Now we have to determine the volume of water is needed to recrystallize 0.900 g of benzoic acid.
From this we conclude that,
As, 68.0 grams of benzoic acid soluble in 1 L of water.
So, 0.900 grams of benzoic acid soluble in
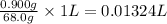 of water.
of water.
The volume of water needed = 0.01324 L = 13.24 mL
conversion used : (1 L = 1000 mL)
Therefore, the volume of water needed to recrystallize 0.900 g of benzoic acid is 13.24 mL.