Answer:
The whole molecule is polar because Sulfur has lone pairs but Carbon doesn't. Lone pairs count more toward polarity, shifting dipole toward S.
Step-by-step explanation:
Even though carbon and sulfur have identical values of electronegativities, the molecule,
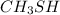 is polar because of the presence of the lone pairs on the sulfur atom.
is polar because of the presence of the lone pairs on the sulfur atom.
The C-S bond is not polar because the both the atoms have electronegatiivty. But S has lone pairs which can attract the bond pairs of the bond between the S and H and thus acquires slightly negative charge and H acquires slightly positive charge.