Answer:
109.8 nm
Step-by-step explanation:
The first ionization energy of a hydrogen atom is,
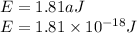
And Plank's constant,
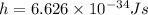
And the speed is,
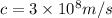
Now the formula for energy will be,

Put all the variable after rearranging for lambda.
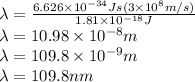
Therefore the wavelength of light is 109.8 nm to ionize a carbon atom .