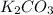Answer:
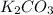
Step-by-step explanation:
Number of moles of K = 0.104 moles
Number of moles of C = 0.052 moles
Number of moles of O = 0.156 moles
The smallest of all is 0.052
So, finding the simplest ratio as:
Number of moles of K = 0.104 / 0.052 = 2
Number of moles of C = 0.052 / 0.052 = 1
Number of moles of O = 0.156 / 0.052 = 3
Thus, the empirical formula is: