Answer:
The volume of the argon at STP is 228.95 mL.
Step-by-step explanation:
Given that,
Initial volume = 205 mL
Initial temperature = -44.0 °C = 229\ K
Final temperature = 273 K
Initial pressure = 712 mm Hg
Final pressure = 760 mm Hg
We need to calculate the volume of the argon at STP
Using gas law
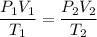
Put the value into the formula
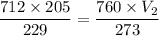
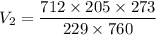
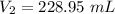
Hence, The volume of the argon at STP is 228.95 mL.