Answer:
0.614 nm
Step-by-step explanation:
Energy of the nth state of one dimensional infinite wall is,
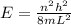
Given the energy to excite an electron from ground state to first excited state is,
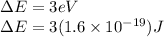
And the Plank's constant is,
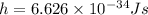
Mass of electron,

Now the energy will of a 1 dimensional infinite wall which excite an electron from ground state to first excited state will be,
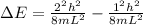
Put all the variables in above equation and rearrange it for L.
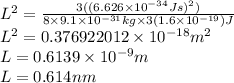
Therefore the width of the box is 0.614 nm.