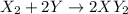Answer:
Gas X
Step-by-step explanation:
The given reaction can be written in the form of chemical equation as shown below as:
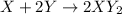
According to law of conservation of mass, the moles of each substance in the reaction must be equal on both reactant and product side.
Also, the question asks for the gas which is diatomic.
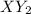 cannot be diatomic as the formula contains 3 atoms.
cannot be diatomic as the formula contains 3 atoms.
Between gas X and gas Y , X has to be diatomic for the reaction to balance as: