Answer:
5.82 L
Step-by-step explanation:
Using Ideal gas equation for same mole of gas as
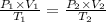
Given ,
V₁ = 2.25 L
V₂ = ?
P₁ = 2.7 atm
T₁ = 12 ºC
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T₁ = (12 + 273.15) K = 285.15 K
At STP, Pressure = 1 atm and Temperature = 273.15 K
So,
P₂ = 1 atm
T₂ = 273.15 K
Using above equation as:
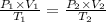

Solving for V₂ , we get:
V₂ = 5.82 L