Answer:
The internal energy of the system is 42 J.
Step-by-step explanation:
Given that,
Heat energy = 82 kJ
Work done = 40 kJ
We need to calculate the change in internal energy
Using first law of thermodynamics
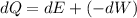
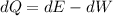
Where, dQ = change in internal energy
dW= Work done
dE= Heat energy
Put the value into the formula
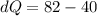
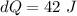
Hence, The internal energy of the system is 42 J.