Answer:
The solution will precipitate.
Step-by-step explanation:
For a solid to precipitate, its ionic product should be more than the solubility product.
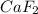 will form its respective ions in the solution as:
will form its respective ions in the solution as:
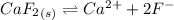
Ionic product =
![[Ca^(2+)][F^-]^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/5nhc4v5bnmg3kg4tn2dnwc1wbbvercllia.png)
Given that:
![[Ca^(2+)]=0.049\ M](https://img.qammunity.org/2020/formulas/chemistry/high-school/eu03dta6whz1d2t242dxu0kwxg9yhqv9c7.png)
![[F^-]=0.147\ M](https://img.qammunity.org/2020/formulas/chemistry/high-school/rao6paccrblfwlk23ft3jipvke81swy5d5.png)
So,
Ionic product = 0.049 × (0.147)² = 0.00106
Given =
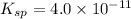
Ionic product > Solubility product
The solution will precipitate.