Answer:
The minimum mass of ethane that could be left over by the chemical reaction is zero.
Step-by-step explanation:
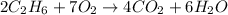
Moles of ethane =
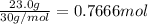
Moles of oxygen gas :
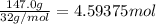
Since, 2 moles of ethane gas react with 7 moles of oxygen gas.
Then 0.7666 mol of ethane gas will react wit:
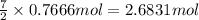
This means that ethane gas is in limited amount.hence ethane is a limiting reagent. Where as oxygen in present in excessive amount.
All the moles ethane gas will go under combustion reaction leaving zero moles behind.