Answer:
Weight of the iceberg is
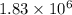 metric ton
metric ton
Step-by-step explanation:
Molar mass of
 = 18 g
= 18 g
So, 6.01 kJ of heat energy is needed to melt 18 g of ice
Hence,
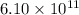 kJ of heat energy is needed to melt
kJ of heat energy is needed to melt
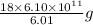 of ice or
of ice or
 g of ice
g of ice
Now,
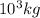 = 1 metric ton
= 1 metric ton
So,
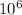 g = 1 metric ton ( 1 kg = 1000 g)
g = 1 metric ton ( 1 kg = 1000 g)
Hence,
 g =
g =
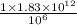 metric ton =
metric ton =
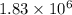 metric ton
metric ton
So, weight of the iceberg is
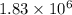 metric ton
metric ton