Answer:
The temperature change from the combustion of the glucose is 6.097°C.
Step-by-step explanation:
Benzoic acid;
Enthaply of combustion of benzoic acid = 3,228 kJ/mol
Mass of benzoic acid = 0.570 g
Moles of benzoic acid =
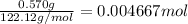
Energy released by 0.004667 moles of benzoic acid on combustion:
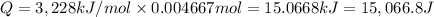
Heat capacity of the calorimeter = C
Change in temperature of the calorimeter = ΔT = 2.053°C
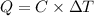
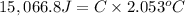
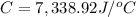
Glucose:
Enthaply of combustion of glucose= 2,780 kJ/mol.
Mass of glucose=2.900 g
Moles of glucose =
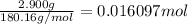
Energy released by the 0.016097 moles of calorimeter combustion:
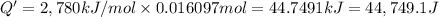
Heat capacity of the calorimeter = C (calculated above)
Change in temperature of the calorimeter on combustion of glucose = ΔT'
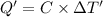
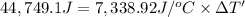
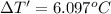
The temperature change from the combustion of the glucose is 6.097°C.