Answer:
pH of the solution is 7.8
Step-by-step explanation:
Acid-base indicators show different colours in their protonated (acid form) and deprotonated from (Basic form or salt).
An acid-base indicator dissociates like weak acid as:
HIn <=> Ln- + H+
pH is calculated using Henderson-Hasselbalch equation,
![pH = p_(ka) +log([Salt])/([Acid])](https://img.qammunity.org/2020/formulas/chemistry/college/smoe1hdmbp46p3mpsgnyaoiwjqwvfppacz.png)
Hln = Protonated form (Acid)
ln- = Deprotonated form (Salt)
Given,
Initial concentration of acid = 0.001 M
Protonated form [Hln] = 0.0002 M
Deprotonated form [ln-] = 0.001 - 0.0002 = 0.0008 M
pKa = 7.2
Now, put the values in Henderson-Hasselbalch equation
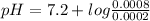
pH = 7.2 + log4
pH = 7.2 + 0.2020
pH = 7.8