Answer:
3.62 moles
Step-by-step explanation:
The decomposition reaction of hydrogen peroxide is shown below as:
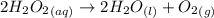
Given that :
Moles of hydrogen peroxide = 7.24 moles
From the reaction,
2 moles of hydrogen peroxide on reaction forms 1 mole of oxygen gas
Also,
1 mole of hydrogen peroxide on reaction forms 1/2 mole of oxygen gas
So,
7.24 moles of hydrogen peroxide on reaction forms
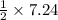 mole of oxygen gas
mole of oxygen gas
Moles of oxygen gas formed = 3.62 moles