Answer:
- The expression of concentration that provides the moles of solute per kilograms of solvent is molality.
- A solution is made up of 0.15 grams of sodium chloride in 1 liter of water. For this solution, the solvent is water.
- A solution is made up of 0.15 grams of sodium chloride in 1 liter of water. For this solution, the solute is sodium chloride.
- If you place 5 moles of sodium chloride and 4 moles of sucrose into 11 moles of water, the molar fraction of sodium chloride would be 0.25.
- A way to express concentration that provides the moles of solute per liter of solution is molarity.
Step-by-step explanation:
Hello,
This is about a concentration units set of questions spelled out as shown below:
- The expression of concentration that provides the moles of solute per kilograms of solvent is molality.
In this case, molality (
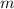 ) is defined as the ratio between the moles of the solute and the mass of the solvent in kilograms only:
) is defined as the ratio between the moles of the solute and the mass of the solvent in kilograms only:
/(kg)](https://img.qammunity.org/2020/formulas/chemistry/college/1poqz59suysimc1ubya3m6jbmojcya3aau.png)
- A solution is made up of 0.15 grams of sodium chloride in 1 liter of water. For this solution, the solvent is water.
In this case, since the sodium chloride is both naturally solid and ionic, it tends to dissociate in aqueous solution which works as a media to allow the solution process, that is why water is the solvent.
- A solution is made up of 0.15 grams of sodium chloride in 1 liter of water. For this solution, the solute is sodium chloride.
In this case, and considering the previous statement, since the molecules of the sodium chloride are dissociated when added to the water (solvent) it is considered as the solute.
- If you place 5 moles of sodium chloride and 4 moles of sucrose into 11 moles of water, the molar fraction of sodium chloride would be 0.25.
In this case, the molar fraction is computed via the ratio between the moles of the sodium chloride and the total moles as shown below:
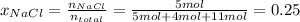
- A way to express concentration that provides the moles of solute per liter of solution is molarity.
In this case, molarity (
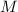 ) is defined as the ratio between the moles of the solute and the volume of the solution in liters only:
) is defined as the ratio between the moles of the solute and the volume of the solution in liters only:
/(L)](https://img.qammunity.org/2020/formulas/chemistry/college/6imsytc19485j23e2d7jsp9zn4e4xhjnzv.png)
Best regards.