Answer:
618.5 min
Step-by-step explanation:
rate of flow of heat =
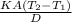
Where K is thermal conductivity of separating medium , D is thickness of separation, A is surface area and T₂-T₁ is temperature difference.
Substituting the values given in the problem
we get
Rate of flow
=
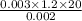
= 36 J m⁻¹ k⁻¹ s⁻¹
Heat energy required to melt ice
= mass x latent heat of fusion
= 4 x 334000 = 1336000 J
Time required
= heat energy required / rate of flow of heat
=
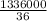
= 618.5 min