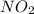The question is incomplete, the complete question is:
At a certain temperature, the equilibrium constant
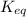 for the following reaction is 0.74:
for the following reaction is 0.74:
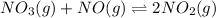
Suppose a 49.0 L reaction vessel is filled with 1.5 mol of
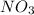 and 1.5 mol of NO. What can you say about the composition of the mixture in the vessel at equilibrium?
and 1.5 mol of NO. What can you say about the composition of the mixture in the vessel at equilibrium?
A. There will be very little
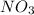 and NO.
and NO.
B. There will be very little
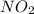
C. Neither of the above is true.
Answer: The correct option is B. there will be very little
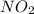
Step-by-step explanation:
We are given:
Initial moles of
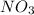 = 1.5 moles
= 1.5 moles
Initial moles of NO = 1.5 moles
Volume of vessel = 49 L
As, moles of reactants and moles of products are equal, the volume term will not appear in the equilibrium constant expression.
Equilibrium constant for the reaction = 0.74
For the given chemical equation:
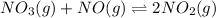
Initial: 1.5 1.5 -
At eqllm: 1.5-x 1.5-x 2x
The expression of equilibrium constant for the above reaction:
![K_(eq)=([NO_2]^2)/([NO_3]* [NO])](https://img.qammunity.org/2022/formulas/chemistry/college/39imzpicwnoq9g872aj55b7gdt9kkmbwcf.png)
Putting values in above equation, we get:
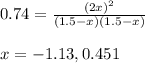
Neglecting the negative value of equilibrium constant because concentration cannot be negative
Equilibrium concentration of
 = (1.5 - x) = (1.5 - 0.451) = 1.049 moles
= (1.5 - x) = (1.5 - 0.451) = 1.049 moles
Equilibrium concentration of NO = (1.5 - x) = (1.5 - 0.451) = 1.049 moles
Equilibrium concentration of
 = 2x =
= 2x =
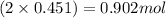
There are 3 possibilities:
- If
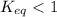 , the reaction is reactant favored
, the reaction is reactant favored - If
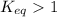 , the reaction is product favored.
, the reaction is product favored. - If
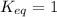 , the reaction is in equilibrium.
, the reaction is in equilibrium.
Here, the value of
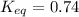 , which is less than 1, therefore the reaction is reactant favored and we can say that there will be very little
, which is less than 1, therefore the reaction is reactant favored and we can say that there will be very little
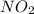 .
.
Hence, the correct option is B. there will be very little