Answer:
250mL
Step-by-step explanation:
The first step in this kind of question is to balance the chemical equation:
Na₂CO₃ + 2HCl → CO₂ + H₂O + 2NaCl
The question asks to figure out how many mL of the reactant Na₂CO₃ are required to produce 11.2 L of CO₂ . To solve this we will use the molar ratio between these two chemical species, that is how many moles of Na₂CO₃ are required to produce one mol of CO₂. We answer this by looking at the equation: the molar ratio is 1 to 1.
So first we need to convert the 11.2 L at STP of CO₂ to moles. CO₂ is a gas and STP means standard temperature and pressure (0°C and 1 atm). The volume of one mol of gas at STP is 22.4 L. So we can convert the 11.2 L to moles using this value:
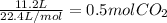
Now that we know how many moles of CO₂ are produced, we can calculate the moles of Na₂CO₃ required using the molar ratio 1:1. To produce one mol of CO₂ we need one mol of Na₂CO₃, hence to produce 0.5 moles of CO₂ we need 0.5 moles of Na₂CO₃.
Now, the question asks how many mL of a 2M solution of Na₂CO₃ are required. M stands for molarity which is a concentration unit meaning moles per liter, hence, 2M means 2 moles per liter of solution (which means in 1000mL of solution there are 2 moles of Na₂CO₃ because 1L=1000mL). So knowing this we must calculate in how many mL of the solution there are 0.5 moles of Na₂CO₃:
0.5 mol Na₂CO₃ ×
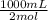 = 250 mL
= 250 mL
250 mL of a 2M solution of Na₂CO₃ are required to produce 11.2 L of CO₂ at STP