Answer:
0.20 moles of propane would be needed to produce 0.60 moles of carbon dioxide.
Step-by-step explanation:
Molecular formula of propane is
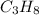
Balanced chemical reaction:
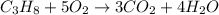
According to balanced reaction-
3 moles of
 are produced from 1 mol of propane
are produced from 1 mol of propane
Hence, 0.60 moles of
 are produced from
are produced from
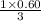 moles of propane or 0.20 moles of propane
moles of propane or 0.20 moles of propane
Therefore, 0.20 moles of propane would be needed to produce 0.60 moles of carbon dioxide.