Answer:
Molecular Mass of insulin = 5004
Step-by-step explanation:
Mass of insulin = 62.4 g
Volume of the solution = 1.00 L
Osmotic pressure = 0.305 L
Temperature = 25 °C = 25 + 273 = 298 K
PV = nRT
Where,
P = osmotic pressure
V = Volume of the solution
n = No. of moles
R gas constant = 0.08206 L atm K^{-1} Mol^{-1}
T = Temperature
PV = nRT
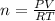
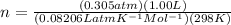
n = 0.01247
No. of mol = Mass in g/molecular mass
Molecular mass = Mass in g/No. of mol
Molecular mass = 62.4/0.01247
= 5004