Answer:
0.549 M
Step-by-step explanation:
Considering:

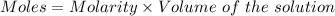
Barium chloride will furnish chloride ions as:
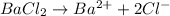
Given :
For barium chloride :
Molarity = 0.240 M
Volume = 28.7 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 28.7×10⁻³ L
Thus, moles of chlorine furnished by barium chloride is twice the moles of barium chloride as shown below:

Moles of chloride ions by barium chloride = 0.013776 moles
Chromium(II) chloride will furnish chloride ions as:
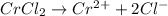
Given :
For chromium(II) chloride :
Molarity = 0.331 M
Volume = 17.5 mL
The conversion of mL to L is shown below:
1 mL = 10⁻³ L
Thus, volume = 17.5×10⁻³ L
Thus, moles of chlorine furnished by chromium(II) chloride is twice the moles of chromium(II) chloride as shown below:

Moles of chloride ions by chromium(II) chloride = 0.011585 moles
Total moles = 0.013776 moles + 0.011585 moles = 0.025361 moles
Total volume = 28.7×10⁻³ L + 17.5×10⁻³ L = 46.2×10⁻³ L
Concentration of chloride ions is:

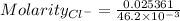
The final concentration of chloride anion = 0.549 M