Answer:
0.2349 moles, 10.3356 g
Step-by-step explanation:
The given reaction is :
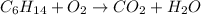
The balanced reaction by equating the same number of each atom both side is :
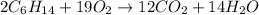
Given that :
Amount of oxygen gas = 11.9 g
Molar mass of oxygen gas = 32 g/mol
The formula for the calculation of moles is shown below:

Thus, moles are:
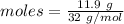
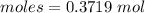
From the reaction,
19 moles of oxygen gas on reaction forms 12 moles of carbon dioxide
Also,
1 mole of oxygen gas on reaction forms 12/19 moles of carbon dioxide
So,
0.3719 moles of zinc on reaction forms
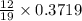 mole of carbon dioxide
mole of carbon dioxide
Moles of carbon dioxide formed = 0.2349 moles
Mass of carbon dioxide = moles×Molar mass
Molar mass of carbon dioxide = 44 g/mol
Mass of carbon dioxide formed = 0.2349 ×44 g = 10.3356 g