Answer:
chlorine gas, sodium hydroxide, and hydrogen gas
Step-by-step explanation:
The electrolysis of the sodium chloride NaCl solution (brine) will produce chlorine Cl₂ gas and solid sodium Na.
After that, because the reaction takes place in water solution, metallic sodium will react with water forming sodium hydroxide and hydrogen gas.
Na + H₂O → NaOH +
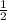 H₂
H₂