Answer:
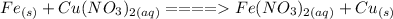
Step-by-step explanation:
Iron is more reactive than copper so if solid iron is introduced into a beaker containing copper nitrate solution, iron will displace copper from the solution forming solid copper while iron enters into solution as ions.
In the second case, if copper is introduced to a solution of iron, there won't be any reaction because copper is less reactive than copper and therefore will not displace iron from a solution of it's salts