Answer:
The mass of the ice is 3.44 kg.
Step-by-step explanation:
Given that,
Area of ice box A= 0.950 m²
Thickness d= 2.50 cm
Temperature T₁= 0° C
Temperature T₂= 35.0°C
Time t = 1 day = 86400 s
We need to calculate the mass of ice
Using equation of rat of heat transfer by conduction
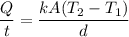
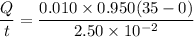
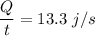
The heat is used to melt the ice
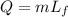
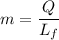 ....(I)
....(I)
We need to calculate the heat
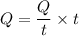
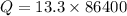
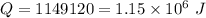
Put the value of Q in the equation (I)
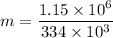
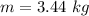
Hence, The mass of the ice is 3.44 kg.