Answer:
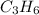
Step-by-step explanation:
Hello!
In this case, since the empirical formula is the smallest representation of the molecular formula, it is known that the times in which the empirical formula is into the molecular formula is a whole number and is computed by dividing the molar mass of the molecular formula by that of the empirical formula as shown below:
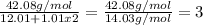
Thus, the molecular formula times the empirical formula by 3 to obtain:
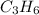
Regards!