Step-by-step explanation:
Let the volume of the solution be 100 ml.
As the volume of glycol = 50 = volume of water
Hence, the number of moles of glycol =
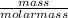
=
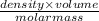
=
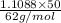
= 0.894 mol
Hence, number of moles of water =
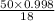
= 2.77
As glycol is dissolved in water.
So, the molality =
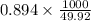
= 17.9
Therefore, the expected freezing point =
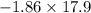
=
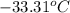
Thus, we can conclude that the expected freezing point is
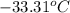 .
.