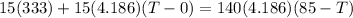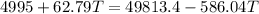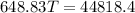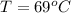Answer:
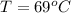
Step-by-step explanation:
Here at thermal equilibrium we can say that thermal energy given by Hot coffee is equal to the thermal energy absorbed by ice cubes
So here we have
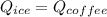
now since ice cubes are added into coffee when it is at melting temperature
So here we can say that final temperature of coffee is T degree C
Now we have
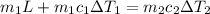
here we have
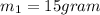
L = 333 kJ/kg = 333 J/g[/tex]
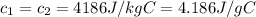
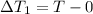
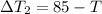
now we have