Answer:
(C) 2
Step-by-step explanation:
Methanol is an organic compound which burns with oxygen to produce carbon dioxide and water.
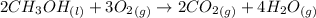
Multiply coefficients times subscripts for each element in the formula, We get that there are 2 number of atoms of the carbon, 8 number of atoms of the hydrogen, and 8 number of atoms of the oxygen on both sides of equation. Hence, it is balanced.
The coefficient of methanol from the balanced reaction is 2.